Research Ethics and Governance
If you have problems with the links below, view our Guidance for accessing LJMU SharePoint pages and documents (Word, 2.4MB).
Human participants in research and knowledge exchange projects
Liverpool John Moores University is committed to maintaining high ethical standards in studies undertaken by its staff and students. LJMU staff and PGR student requirements for research ethics and governance are summarised in the flow diagram below and details are provided via:
LJMU Research Ethics & Governance SharePoint
LJMU Research Ethics and Governance Standard Operating Procedures
A favourable ethics opinion from a Research Ethics Committee, obtaining LJMU Research Governance Approval and/or obtaining LJMU sponsorship must be obtained prior to recruiting participants (or collecting data for secondary analysis). Ethical review, governance approval or sponsorship cannot be obtained retrospectively.
Research ethics
Research ethics ensures the safety, dignity and rights of research participants whilst providing assurance that studies are conducted within an ethical framework as outlined in LJMU Code of Practice for Research and Knowledge Exchange. Unless deemed exempt by LJMU, all research or knowledge exchange projects (including pilot studies) led by LJMU staff and students involving human participants or the collections and/or use of their data requires a favourable ethical opinion from an appropriate Research Ethics Committee (REC). If the requisite favourable ethics opinion is not secured, your project may not be covered under the University's liability/indemnity policies. The process of research ethics at LJMU also covers LJMU Research Governance Approval. For projects exempt from LJMU ethical review, there are separate processes to obtain LJMU Research Governance Approval.
Research Governance
Research Governance includes regulations, principles and standards of good practice that exist to achieve and continuously improve research quality. Research governance includes LJMU Research Governance Approval and LJMU Sponsorship of research. If the requisite LJMU research governance approval or LJMU sponsorship is not secured, your project may not be covered under the University's liability/indemnity policies. The process of research ethics at LJMU also covers LJMU Research Governance Approval. For projects exempt from LJMU ethical review, there are separate processes to obtain LJMU Research Governance Approval.
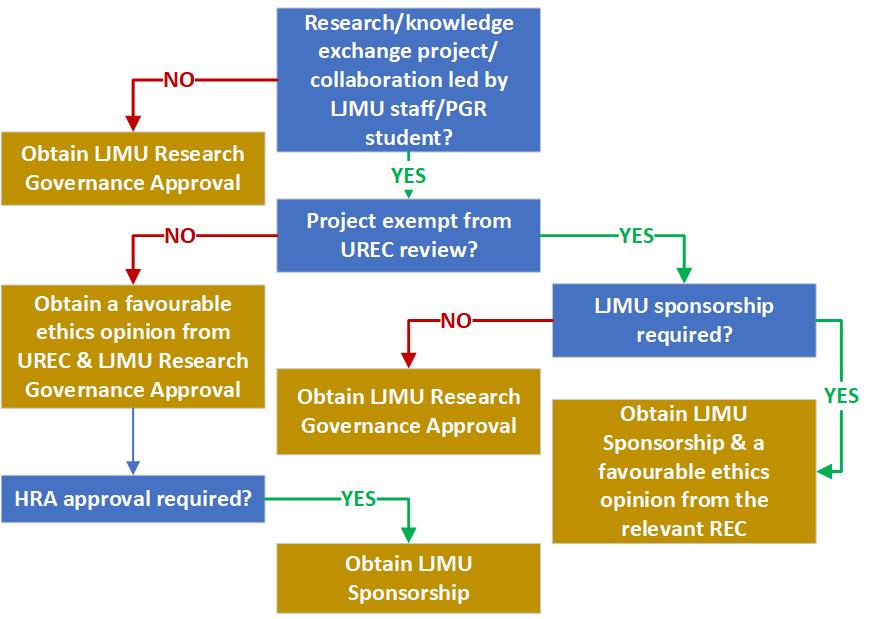
Text description of the flow diagram to summarise the LJMU staff and PGR student requirements for research ethics and governance.
Step One: Is the Research or Knowledge Exchange Project or Collaboration led by LJMU staff or a Postgraduate Research (PGR) student? If yes, go to Step Three. If no, go to Step Two.
Step Two: Obtain LJMU Research Governance Approval.
Step Three: Is the project exempt from University Research Ethics Committee (UREC) review? If yes, go to Step Six. If no, go to Step Four.
Step Four: Obtain a favourable ethics opinion from UREC & LJMU Research Governance Approval and Continue to Step Five.
Step Five: Is HRA approval required? If Yes, go to Step Nine.
Step Six: Is LJMU Sponsorship required? If yes, go to Step Seven. If no, go to Step Eight.
Step Seven: Obtain LJMU Sponsorship and a favourable ethics opinion from the relevant Research Ethics Committee (REC).
Step Eight: Obtain LJMU research Governance Approval.
Step Nine: Obtain LJMU Sponsorship.


