Research Ethics
If you have problems with the links below, view our Guidance for accessing LJMU SharePoint pages and documents (Word, 2.4MB).
Ethical review required?
For guidance, complete the “Do I need ethical approval from a REC?” decision tool. Please note, School/Faculty RECs may require ethical review be conducted on undergraduate or post graduate taught student projects irrespective of the outcome of the “Do I need ethical approval from a REC?” decision tool.
Research ethics training
LJMU research ethics training is mandatory for students applying to UREC for ethical review. Staff applying to UREC for ethical review are invited to complete the training, but the training is not mandatory for staff. School/Faculty RECs may mandate LJMU research ethics training for LJMU undergraduate and post graduate taught student.
Where and how to apply for ethical review
School/Faculty RECs review LJMU undergraduate and post graduate taught student projects that meet the criteria for expedited ethical review. The University Research Ethics Committee (UREC) reviews staff and post graduate research student projects. Some Projects are exempt from LJMU REC review and may require ethical review by a REC external to LJMU. For example, research using NHS/HSC patients/service users as participants require ethical review by an NHS/HSC REC. Projects that require ethical review by an NHS/HSC REC or MODREC require LJMU sponsorship. It should be noted that UREC review and not NHS/HSC review is required if using NHS staff, NHS facilities or data – but LJMU sponsorship is still required. For further information please refer to the LJMU Research Ethics and Governance Standard Operating Procedures. The process for ethics review for projects led by LJMU staff and PGR students is summarised in the flow diagram below.
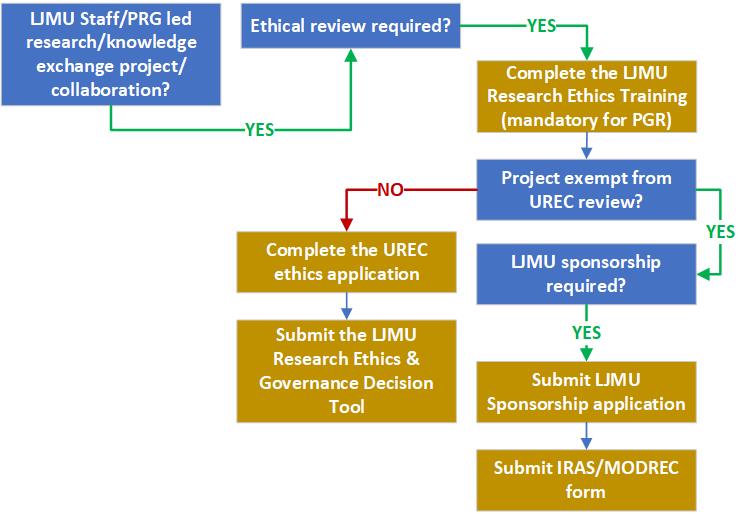
Text description of the flow diagram to summarise process of UREC review.
Step 1. LJMU staff/PGR led research/knowledge exchange project/collaboration? If yes, go to step 2.
Step 2. Is ethical review required? If yes, go to step 3.
Step 3. Complete the LJMU Research Ethics Training (mandatory for PGR students). Continue to step 4.
Step 4. Is the project exempt from UREC review? If yes, go to step 5. If no, go to step 7.
Step 5. Is LJMU Sponsorship required? If yes, go to step 6.
Step 6. Submit the LJMU sponsorship application and submit the IRAS/MODREC form.
Step 7. Complete the UREC ethics application form and submit the LJMU Research Ethics and Governance Decision Tool.


